


Sterile EVA Pillow Bags for Aqueous & Oil-Based Medications – FDA-Cleared for Rx Use
The Sterile Empty EVA Pillow Bag is a pharmacy-grade, single-use container designed for aseptic storage and dispensing of both aqueous and oil-based medications, including injectables, hormones, and specialty compounds. Individually packed and ETO-sterilized, it ensures uncompromised sterility for critical compounding workflows.
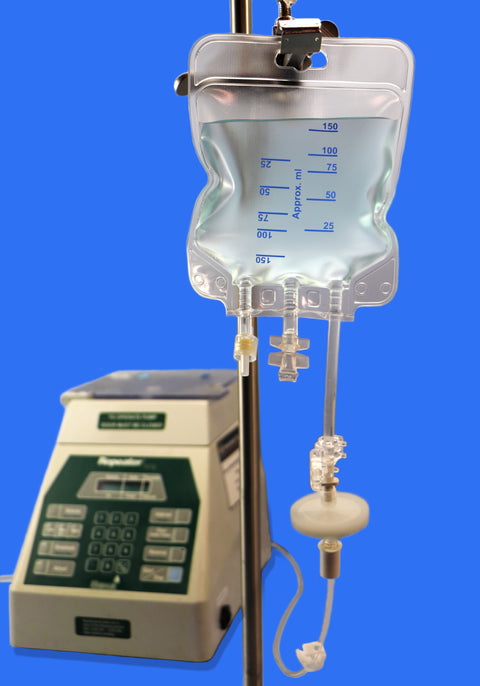
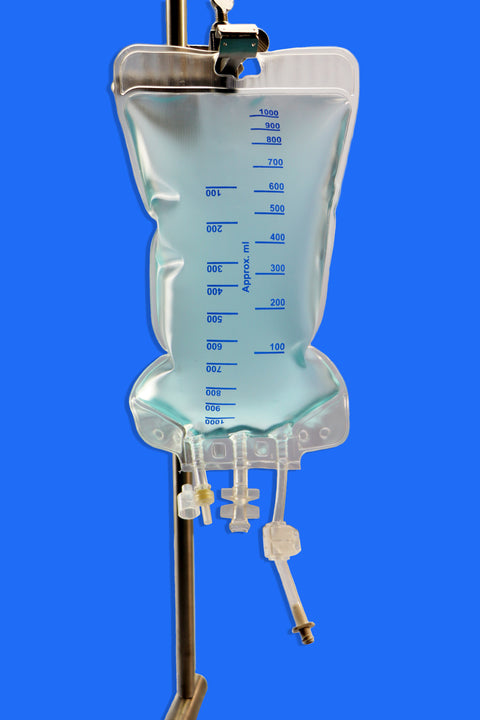
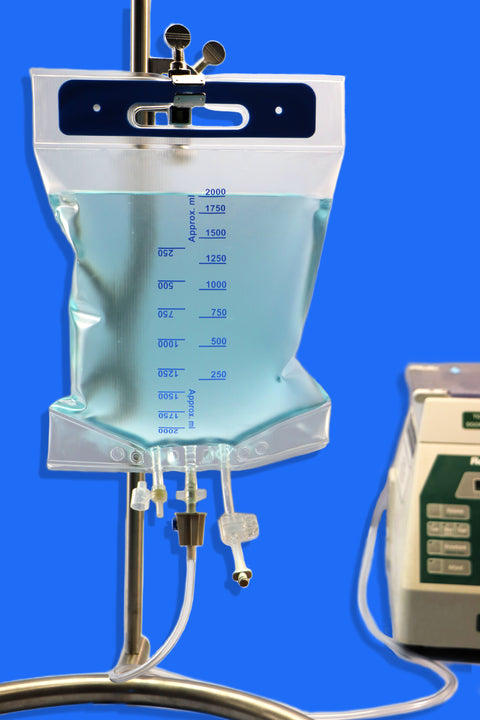
Equipped with three ports, including a female luer lock, the bag integrates easily with repeater pumps, filtration systems, and tube sets for seamless sterile transfers into vials. Available in 150 mL to 5000 mL sizes, with reinforced plates in larger volumes, it offers both flexibility and structural integrity.
FDA-cleared and Rx-qualified, this bag is the trusted choice for pharmacies requiring reliable, sterile fluid handling under controlled conditions.
Relevant Industries
• Sterile Compounding Pharmacies (Primary Use Case)
• Pharmaceutical Manufacturing
• Biotech & Biologics
• Hospital Pharmacies & Infusion Clinics
• Clinical Research Organizations (CROs)
• Sterile Container for Rx and Pharmaceutical Medication
Designed for sterile handling and secure transfer of compounded prescription drugs.
• Sterile Container for Aqueous-Based Medication
Suitable for GLP-1 analogs, Semaglutide, Tirzepatide, vaccines, and injectables.
• Sterile Container for Oil-Based Medication
Compatible with hormone therapies, testosterone, and other oil-based solutions.
• Precise Dispensing into Sterile Vials
Supports accurate vial filling using repeater pumps under aseptic conditions.
• Seamless Integration with Sterile Filtration
Connects with filtration systems for closed, contamination-free transfer and storage.
• Luer Lock Connection for Easy Compatibility
Ensures secure interface with filters, tubing sets, and IV systems.
• FDA Cleared for Rx Use — Complies with U.S. medical device regulations for intravenous fluid containers.
• Designed for Aseptic Handling — Intended for use by trained professionals in sterile compounding environments.
• Single-Use, Non-Resterilizable — Ensures safety, sterility, and regulatory compliance with every use.
• Caution: Many EVA bags on the market lack FDA clearance and are not qualified for Rx use—choose only FDA-cleared products for sterile pharmaceutical applications.
• Volume Capacity: Available in sizes ranging from 150 mL to 5000 mL
• Ports: Equipped with 3 ports, including one Luer Lock (LF) port for secure, leak-free connections
• Strengthening Plate: Included in sizes ≥ 2000 mL to ensure structural durability
• Material Composition: Latex-free, DEHP-free, non-toxic, and pyrogen-free
• Sterilization Method: Sterilized using Ethylene Oxide (ETO) for validated sterility
• Design Features:
1. Injection port for supplement addition
2. Spike port for aseptic medication transfer
3. Inviolable clamp to secure contents and maintain sterility
• Compatibility: Seamlessly integrates with sterile filtration systems and repeater pumps for precise dispensing
• Regulatory Status: FDA Cleared for Rx Use; meets U.S. medical device quality and safety standards
• Sterility Assurance Level (SAL): Validated to 10⁻⁶ per ISO 11135
• Shelf Life: 5 years from sterilization date; packaging maintains a sterile barrier through shipping and handling
• Biocompatibility Testing:
1. Passed In Vitro Cytotoxicity (ISO 10993-5:2009)
2. Passed Sensitization & Intracutaneous Reactivity (ISO 10993-10:2010)
3. Passed Haemolysis Test (ASTM F756-13)
4. Passed Hemocompatibility (ISO 10993-4:2009)
• Sterilization Residuals: Ethylene oxide residuals ≤4 mg, compliant with ISO 10993-7:2008
• Endotoxin & Pyrogen Testing:
1. Passed Bacterial Endotoxins Test (USP <85>)
2. Passed Pyrogen Test (USP <151>)
• Systemic Toxicity: Passed Rabbit Pyrogen Test (ISO 10993-11:2017)
• Transfusion & Infusion Assemblies: Compliant with USP <161>
