


5 L Sterile EVA Bag (Individually Packed) for Rx Use
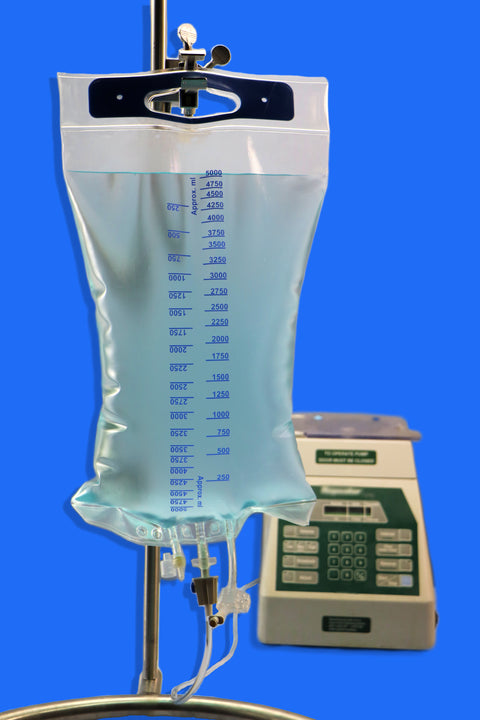
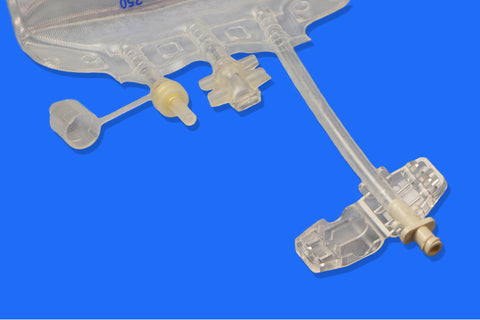
Sterile Empty EVA Pillow Bag, 5 L / 5000 mL, 3 Ports, With One Luer Lock Port, For Rx Use
Product must be purchased in packs of 5
Relevant Industries
• Sterile Compounding Pharmacies (Primary Use Case)
• Pharmaceutical Manufacturing
• Biotech & Biologics
• Hospital Pharmacies & Infusion Clinics
• Clinical Research Organizations (CROs)
• Sterile Container for Rx and Pharmaceutical Medication
Designed for sterile handling and secure transfer of compounded prescription drugs.
• Sterile Container for Aqueous-Based Medication
Suitable for GLP-1 analogs, Semaglutide, Tirzepatide, vaccines, and injectables.
• Sterile Container for Oil-Based Medication
Compatible with hormone therapies, testosterone, and other oil-based solutions.
• Precise Dispensing into Sterile Vials
Supports accurate vial filling using repeater pumps under aseptic conditions.
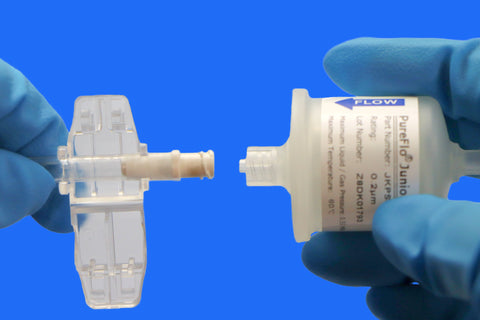
• Seamless Integration with Sterile Filtration
Connects with filtration systems for closed, contamination-free transfer and storage.
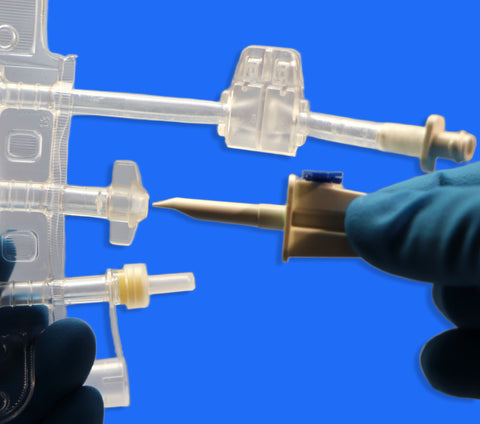
• Luer Lock Connection for Easy Compatibility
Ensures secure interface with filters, tubing sets, and IV systems.
• FDA Cleared for Rx Use — Complies with U.S. medical device regulations for intravenous fluid containers.
• Designed for Aseptic Handling — Intended for use by trained professionals in sterile compounding environments.
• Single-Use, Non-Resterilizable — Ensures safety, sterility, and regulatory compliance with every use.
• Caution: Many EVA bags on the market lack FDA clearance and are not qualified for Rx use—choose only FDA-cleared products for sterile pharmaceutical applications.
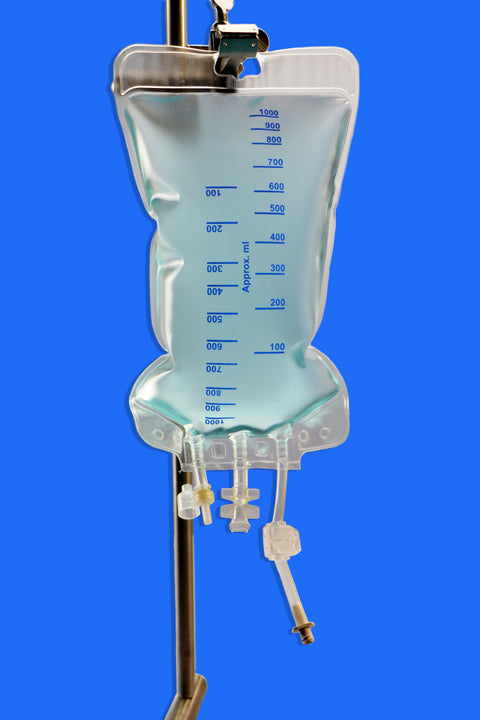
• Volume Capacity: Available in sizes ranging from 150 mL to 5000 mL
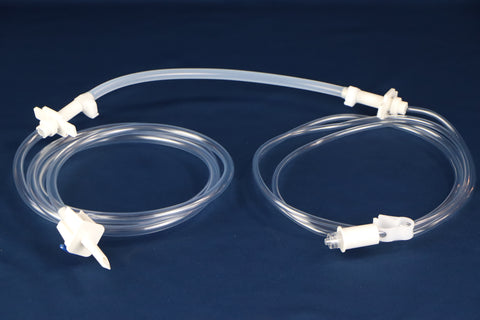
• Ports: Equipped with 3 ports, including one Luer Lock (LF) port for secure, leak-free connections
• Strengthening Plate: Included in sizes ≥ 2000 mL to ensure structural durability
• Material Composition: Latex-free, DEHP-free, non-toxic, and pyrogen-free
• Sterilization Method: Sterilized using Ethylene Oxide (ETO) for validated sterility
• Design Features:
1. Injection port for supplement addition
2. Spike port for aseptic medication transfer
3. Inviolable clamp to secure contents and maintain sterility
• Compatibility: Seamlessly integrates with sterile filtration systems and repeater pumps for precise dispensing
• Regulatory Status: FDA Cleared for Rx Use; meets U.S. medical device quality and safety standards
• Sterility Assurance Level (SAL): Validated to 10⁻⁶ per ISO 11135
• Shelf Life: 5 years from sterilization date; packaging maintains a sterile barrier through shipping and handling
• Biocompatibility Testing:
1. Passed In Vitro Cytotoxicity (ISO 10993-5:2009)
2. Passed Sensitization & Intracutaneous Reactivity (ISO 10993-10:2010)
3. Passed Haemolysis Test (ASTM F756-13)
4. Passed Hemocompatibility (ISO 10993-4:2009)
• Sterilization Residuals: Ethylene oxide residuals ≤4 mg, compliant with ISO 10993-7:2008
• Endotoxin & Pyrogen Testing:
1. Passed Bacterial Endotoxins Test (USP <85>)
– Passed Pyrogen Test (USP <151>)
• Systemic Toxicity: Passed Rabbit Pyrogen Test (ISO 10993-11:2017)
• Transfusion & Infusion Assemblies: Compliant with USP <161>
Need technical support or help with a quote? Contact Us.