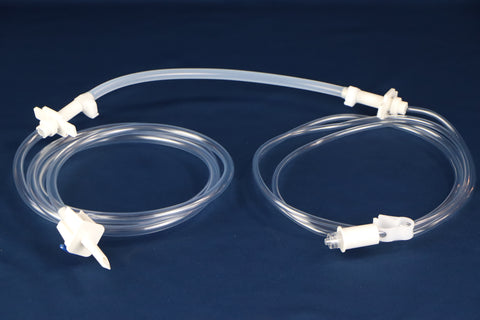
PureFlo® JKP Capsule Filter 0.65um / 0.2um - Sterile Compounding (Aqueous)
PureFlo JKP Series Capsule, Highly Asymmetric Dual Layer PES 0.65μm / 0.2μm PES Sterilizing Grade Hydrophilic Membrane, Effective Filtration Area 260 cm2, Luer Loc Female Inlet, Luer Loc Male Outlet, Factory sterilized by ETO
Relevant Industries
• Sterile Compounding
Applications
• Sterilizing Grade Filtration
• Bacteria Removal Filtration
• Aqueous Base Solution
• Vaccine
• Injectables
• GLP-1 / Semaglutide / Tirzepatide
Application: Sterile Compounding, Pharmaceutical, Biological
Membrane Material: PES Sterilizing Grade (Hydrophilic, Low Protein Binding, High Flow Rate)
Pore Size: Highly Asymmetric Dual Layer 0.65μm / 0.2μm
Suitable Solution: Aqueous & Alcohol Base
Estimated Filtration Volume*: 3.36L - 14.5L
Effective Filtration Area: 260 cm²
Sterilization: Factory Sterilized by ETO
Inlet Connection: Luer Loc Female
Outlet Connection: Luer Loc Male
Hold-up / Retention Volume: 8.6 mL (Approx.)
Bacterial Retention: Retains Brevundimonas diminuta (≥10^7 CFU/cm²), consistent with ASTM F838-20
Sterilization: ETO, complies with ISO 10993-7:2008, ANSI/AAMI/ISO 11135, SAL of 10^-6
USP Compliance: Compliant with USP <797>
Material Compliance: 21CFR Part 177, USP Class VI
Endotoxins: <0.25 EU/ml (LAL Test)
Usage: Safe for human and veterinarian use
cGMP Compliance: CFR part 210 & 211, 21 CFR part 600, 21 CFR part 680
Animal Components: Free from animal-derived materials, TSE/BSE free
Need technical support or help with a quote? Contact Us.