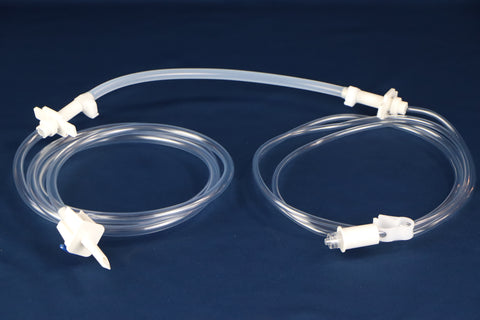
PureFlo® MKP Capsule Filter 1.0um - Sterile Compounding (Oil)
PureFlo® MKP Series Capsule, 1.0 um PTFE Hydrophobic Membrane, Effective Filtration Area 500 cm2, Luer Lock Female Inlet, Luer Lock Male Outlet, Factory sterilized by ETO
Relevant Industries
• Sterile Compounding
Applications
• Bacteria Removal Filtration
• Oil Base Solution
• DMSO / Organic Compound
• Injectables
• Testosterone
• Application: Sterile Compounding, Pharmaceutical, Biological
• Membrane Material: PTFE (Hydrophobic, Chemically Inert, High Temperature Resistance)
• Pore Size: Single Layer 1.0μm
• Suitable Solution: Oil & Alcohol Base
• Estimated Filtration Volume*: 32.5L – 132.5L (50% less for Oils)
• Effective Filtration Area: 500 cm²
• Sterilization: Factory Sterilized by ETO
• Inlet Connection: Luer Lock Female
• Outlet Connection: Luer Lock Male
• Hold-up / Retention Volume: 47 mL (Approx.)
• Integrity Test / Bubble Point: ≥ 5 psi in 60% IPA/40% Water
• Sterilization: ETO, complies with ISO 10993-7:2008, ANSI/AAMI/ISO 11135, SAL of 10^-6
• USP Compliance: Compliant with USP <797>
• Material Compliance: 21CFR Part 177, USP Class VI
• Endotoxins: <0.25 EU/ml (LAL Test)
• Usage: Safe for human and veterinarian use
• cGMP Compliance: CFR part 210 & 211, 21 CFR part 600, 21 CFR part 680
• Animal Components: Free from animal-derived materials, TSE/BSE free
Need technical support or help with a quote? Contact Us.