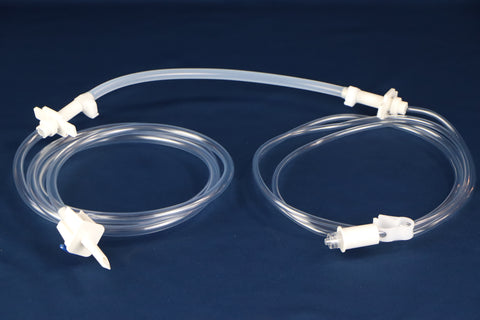
PharMed® BPT Biocompatible Peristaltic Pump Tubing – Bioprocessing
PharMed® BPT Biocompatible Peristaltic Pump Tubing is a premium solution tailored for biopharmaceutical fluid transfer applications. Designed to endure the mechanical stresses of peristaltic pumping, it offers exceptional flex life, ultra-low particulate spallation, and superior chemical resistance. Its biocompatible formulation ensures fluid integrity, while compatibility with clean-in-place (CIP) and steam-in-place (SIP) sterilization systems makes it ideal for high-purity processes. PharMed® BPT tubing is validated to meet stringent regulatory standards, ensuring reliable performance in critical biopharmaceutical operations.
Don't see the Inner or Outer Diameter you're looking for? Contact us for more options
Relevant Industries
• Life Sciences
• Biopharmaceutical Manufacturing
• Biotechnology
• Pharmaceutical Production
• Healthcare and Medical Devices
Applications
• Media Process Systems
• Bioreactor Process Lines
• Peristaltic Pump Tubing Applications with Extended Tubing Life
• Vaccine Manufacturing Pipelines
• Aseptic Filling Operations
• Cell Harvesting
• Shear-Sensitive Fluid Transfer
• Filtration and Fermentation Processes
• Diagnostic Test Product Development
• Biocompatible fluid surface with ultra-low particulate spallation and opaque cream appearance to protect from visible and UV light
• Superior flex life; outperforms silicone tubing in peristaltic pumps with excellent barrier properties and low gas/vapor permeability
• Operates from -59°C to 135°C (-75°F to 275°F); withstands up to 5 autoclave cycles and gamma irradiation up to 50kGy
• High chemical resistance to dilute acids, bases, salts, alcohols, emulsions, and high-purity water
• Durometer Hardness: Shore A, 64 (ASTM D2240); Water Absorption: 0.30% (ASTM D570); Max Temp: 275°F; Embrittlement Temp: -75°F (ASTM D746)
• Available in multiple sizes with varying I.D., O.D., wall thickness, working pressures, and vacuum ratings
Biocompatibility:
Passed USP <87> (in vitro) biological reactivity tests.
Passed USP <88> Class VI (in vivo) biological reactivity tests.
Endotoxin Testing:
Passed USP <85> Limulus Amebocyte Lysate test.
Particulate Testing:
Compliant with USP <788> subvisible particulate standards.
Bioburden Testing:
Passed ISO 11737-1 standards.
Regulatory Compliance:
All formulas are Animal-Derived Component Free.
Validation Guide and Regulatory Information Overview (RIO) available upon request.
Need technical support or help with a quote? Contact Us.