


SKU: D25CS020LFLM-PH-ETO
PureFlo® D25 Disc Filter 0.2um - Sterile Compounding (Aqueous)
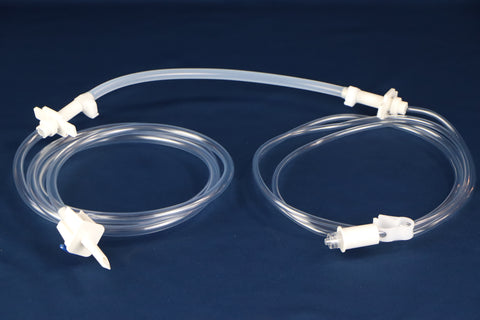
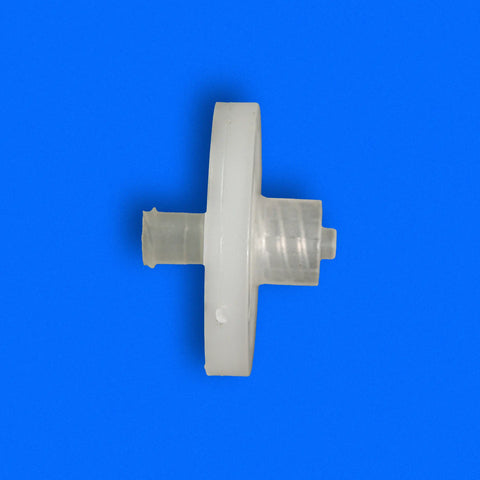
PureFlo D25C Series, 0.2 um PES Sterilizing Grade Hydrophilic Membrane, Effective Filtration Area 4.5 cm2, Luer Loc Female Inlet, Luer Loc Male Outlet, Factory sterilized by ETO
Product must be purchased in sets of five.
Select Title
Out of stock
$9.59
Relevant Industries
• Sterile Compounding
Applications
• Sterilizing Grade Filtration
• Bacteria Removal Filtration
• Aqueous Base Solution
• Vaccine
• Injectables
• GLP-1 / Semaglutide / Tirzepatide
- Application: Sterile Compounding, Pharmaceutical, Biological
- Membrane Material: PES Sterilizing Grade (Hydrophilic, Low Protein Binding, High Flow Rate)
- Pore Size: Single Layer 0.2μm
- Suitable Solution: Aqueous & Alcohol Base
- Estimated Filtration Volume*: 60 mL – 200 mL
- Effective Filtration Area: 4.6 cm²
- Sterilization: Factory
Sterilized by
ETO - Inlet Connection: Luer Loc Female
- Outlet Connection: Luer Loc Male
- Hold-up
Volume: 1.27 mL (Approx.)
- Integrity Test / Bubble Point: ≥18 psi in 60% IPA/40% Water, ≥17.5 psi in 70% IPA/30% Water, & ≥50 psi in 100% DI Water at 22°C
- Bacterial Retention: Retains Brevundimonas diminuta (≥10^7 CFU/cm²)
- Sterilization: ETO, complies with ISO 10993-7:2008, ANSI/AAMI/ISO 11135, SAL of 10^-6
- USP Compliance: Compliant with USP <797>
- Material Compliance: 21CFR Part 177, USP Class VI
- Endotoxins: <0.25 EU/ml (LAL Test)
- Usage: Safe for human and veterinarian use
- CGMP Compliance: CFR part 210 & 211, 21 CFR part 600, 21 CFR part 680
- Animal Components: Free from animal-derived materials, TSE/BSE free
Need technical support or help with a quote? Contact Us.
Viable Options